A Molecular Mailman That Could Transform Neuroscience
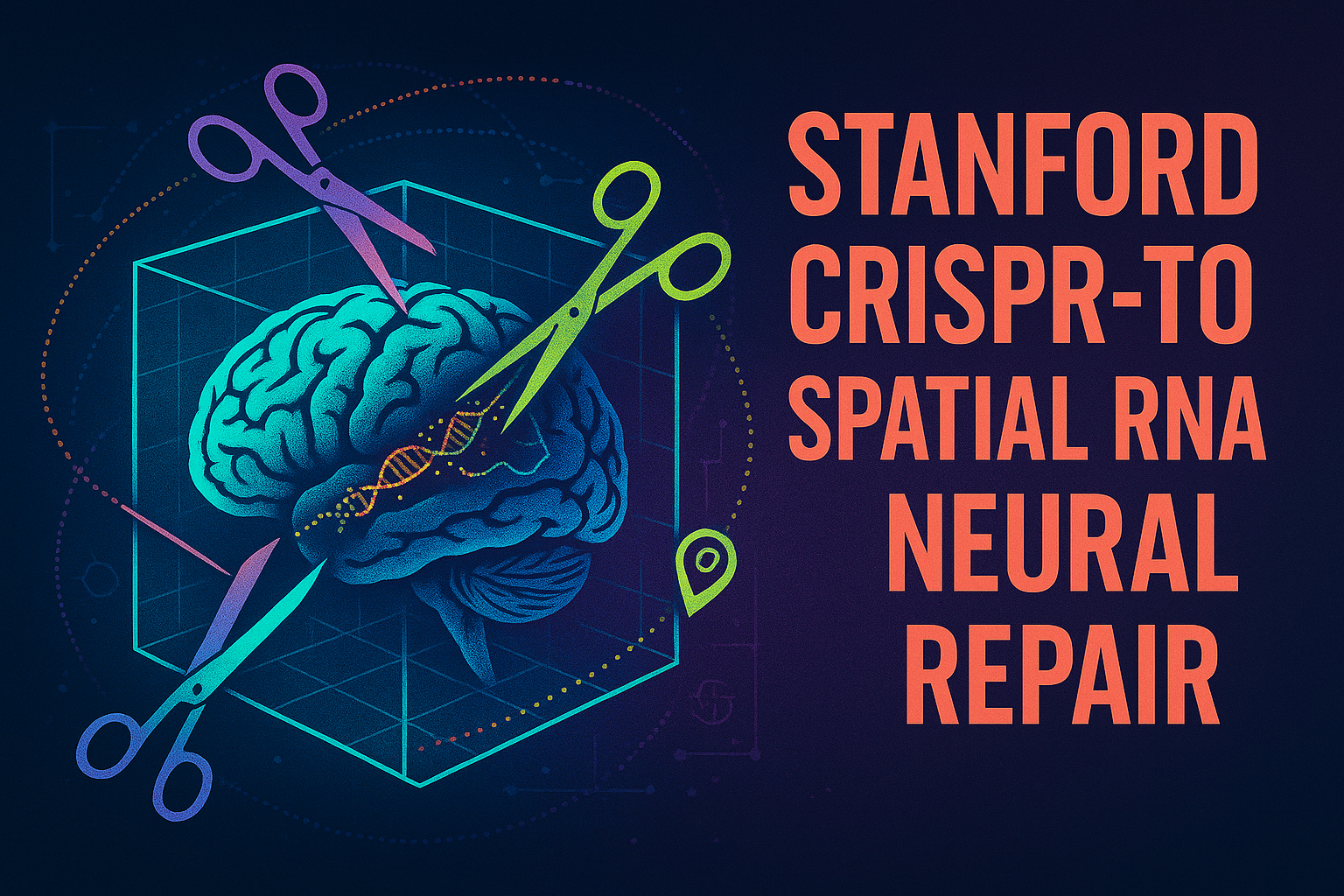
In a groundbreaking achievement that redefines the boundaries of genetic medicine, Stanford University researchers have engineered CRISPR technology to act not as molecular scissors, but as a precision delivery system—a “molecular mailman” capable of transporting therapeutic RNA to exact locations within neurons. Stanford +2 This innovation, published in Nature in May 2024, represents the birth of an entirely new therapeutic class: spatial RNA medicine. Stanford +2
The technology, dubbed CRISPR-TO (CRISPR-mediated Transcriptome Organization), achieved what many thought impossible: delivering RNA molecules to specific “zip codes” within neurons, resulting in up to 50% improvement in neurite growth within just 24 hours. Stanford +2 For millions suffering from neurodegenerative diseases like ALS, Alzheimer’s, and spinal cord injuries, this breakthrough offers unprecedented hope for treatments that work with, rather than against, the body’s natural cellular machinery.
Unlike traditional CRISPR systems that permanently alter DNA, CRISPR-TO represents a fundamental paradigm shift. It harnesses the RNA-targeting capabilities of CRISPR-Cas13 to create a reversible, programmable system for reorganizing cellular RNA— Nature +2opening therapeutic possibilities that were previously confined to the realm of science fiction. Stanford +2
Understanding CRISPR: From Gene Editing to RNA Transport
The Evolution of CRISPR Technology
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) began as nature’s solution to an ancient problem: how bacteria defend themselves against viral invaders. This adaptive immune system, first observed in E. coli in 1987 by Yoshizumi Ishino, remained a scientific curiosity until researchers recognized its potential as a programmable genetic tool. Bitesize Bio +2
The CRISPR revolution truly began in 2012 when Jennifer Doudna and Emmanuelle Charpentier demonstrated that the bacterial immune system could be reprogrammed to target any DNA sequence. This discovery, which earned them the 2020 Nobel Prize in Chemistry, launched a new era in genetic medicine. The Stanford Daily +6 Traditional CRISPR-Cas9 systems work by creating precise cuts in DNA, allowing researchers to delete, insert, or modify genetic sequences. LWW
However, DNA editing comes with inherent risks. Once DNA is altered, the changes are permanent and passed on to daughter cells. In the delicate environment of the nervous system, where neurons must maintain precise connections and functions over decades, permanent genetic modifications carry substantial risks of unintended consequences.
The RNA Revolution: CRISPR-Cas13 Systems
The discovery of CRISPR-Cas13 in 2015 marked a pivotal moment in the field. Nature Unlike Cas9, which targets double-stranded DNA, Cas13 naturally targets single-stranded RNA molecules. BioDesign Research +5 This distinction is crucial for several reasons:
Reversibility: RNA modifications are temporary, as RNA molecules are constantly being produced and degraded within cells. This allows for therapeutic interventions that can be adjusted or discontinued as needed. NCBI
Safety: By avoiding DNA alterations, Cas13 systems eliminate the risk of permanent genomic changes that could lead to cancer or other genetic disorders.
Flexibility: RNA targeting is generally PAM-independent (Protospacer Adjacent Motif), providing broader targeting capabilities than DNA-targeting systems. BioDesign ResearchNCBI
The Cas13 family comprises multiple variants, each with unique properties. Cas13a (LwaCas13a) was the first discovered RNA-targeting CRISPR effector. NCBI Cas13b (PspCas13b) proved highly efficient for mammalian applications. AddgeneTaylor & Francis Cas13d (RfxCas13d), a compact variant suitable for viral delivery, has shown particular promise for neurological applications. Nature +2
Stanford’s Innovation: From Cutting to Carrying
Stanford’s CRISPR-TO system represents a conceptual leap beyond existing CRISPR applications. Rather than using Cas13’s natural RNA-cutting ability, the Stanford team, led by Dr. Stanley Qi and postdoctoral scholar Dr. Mengting Han, engineered the system to transport RNA molecules to specific cellular locations. Welcome to Bio-X +3
The innovation required solving multiple technical challenges. First, the team had to disable Cas13’s nuclease activity while preserving its RNA-binding capability. They achieved this by creating nuclease-dead variants (dCas13) that could still recognize and bind specific RNA sequences but lacked the ability to cleave them. NCBI +3
Next, they engineered localization signals—molecular “addresses”—that direct the Cas13-RNA complex to specific subcellular compartments. These signals exploit the cell’s natural transport machinery, including motor proteins that move cargo along microtubules, to deliver therapeutic RNA precisely where needed. StanfordNeuroscience News
The Science Behind Neural RNA Transport and Repair
Understanding Neuronal Architecture and RNA Localization
Neurons present unique challenges for therapeutic intervention. A single motor neuron can extend over one meter from the spinal cord to muscles in the foot, making it one of the longest cells in the human body. This extraordinary length creates logistical challenges for cellular maintenance and repair. Stanford +2
Within neurons, RNA localization is critical for proper function. Synapses—the connection points between neurons—require local protein synthesis to maintain plasticity and respond to stimulation. This necessitates the transport of specific mRNAs from the cell body to distant synaptic sites. In healthy neurons, sophisticated transport systems ensure that the right RNAs reach the right locations at the right times.
However, in neurodegenerative diseases, this transport system often fails. In ALS, for example, motor neurons lose their ability to transport RNA to synapses and axon terminals. Without local protein synthesis, these distant sites cannot maintain themselves or respond to damage, leading to progressive neuronal dysfunction and death. StanfordNeuroscience News
The Molecular Machinery of RNA Transport
RNA transport in neurons relies on several key components:
RNA-Binding Proteins (RBPs): These proteins recognize specific sequences or structures in mRNAs and package them into transport granules. Dysfunction of RBPs is implicated in numerous neurological diseases.
Motor Proteins: Kinesin and dynein motors move cargo along microtubule tracks that extend throughout the neuron. Kinesin typically moves cargo away from the cell body (anterograde transport), while dynein moves cargo toward it (retrograde transport).
Adaptor Proteins: These molecules link RNA-containing granules to motor proteins, ensuring proper cargo loading and transport directionality.
Local Translation Machinery: Ribosomes and translation factors must also be present at distant sites to produce proteins from transported mRNAs.
Stanford’s CRISPR-TO system ingeniously hijacks this natural transport machinery. By fusing appropriate localization signals to dCas13, the system can direct therapeutic RNAs to ride along existing transport routes to reach damaged or dysfunctional sites within neurons. PubMed
Mechanisms of Neural Repair and Regeneration
The nervous system’s repair capacity varies dramatically between the peripheral nervous system (PNS) and central nervous system (CNS). Peripheral neurons retain significant regenerative ability—after injury, they can regrow axons and reestablish connections. This regeneration depends on a coordinated program of gene expression changes that produce growth-promoting proteins. WikipediaScienceDirect
In contrast, CNS neurons in the brain and spinal cord show limited regenerative capacity in adult mammals. Multiple factors contribute to this limitation: WikipediaScienceDirect
Intrinsic Factors: Adult CNS neurons lose expression of growth-promoting genes and activate growth-inhibiting pathways. They also show reduced metabolic capacity to support the energy-intensive process of axon growth.
Extrinsic Factors: The CNS environment actively inhibits regeneration. Myelin-associated proteins like Nogo, MAG, and OMgp signal neurons to halt growth. Additionally, injury triggers glial scar formation, creating both physical and chemical barriers to regeneration. WikipediaScienceDirect
Recent research reveals that CNS neurons retain greater regenerative potential than previously thought. When provided with appropriate growth signals and a permissive environment, even adult CNS neurons can regenerate. ScienceDirect This discovery underlies the therapeutic potential of CRISPR-TO—by delivering growth-promoting RNAs directly to sites of injury, the technology could unlock latent regenerative capacity.
Stanford’s CRISPR-TO: Key Features and Innovations
Technical Architecture of the System
The CRISPR-TO platform consists of several engineered components working in concert:
Catalytically Inactive Cas13 (dCas13): The foundation of the system is a modified Cas13 protein with mutations in its HEPN (Higher Eukaryotes and Prokaryotes Nucleotide-binding) domains. These mutations eliminate RNA cleavage activity while preserving specific RNA binding through base-pairing with guide RNAs. NCBI +4
Programmable Guide RNAs: Similar to traditional CRISPR systems, CRISPR-TO uses guide RNAs to specify which cellular RNAs to target. The guide RNA sequence determines binding specificity through complementary base pairing with target RNAs.
Modular Localization Signals: The true innovation lies in the addition of subcellular localization signals to dCas13. The Stanford team created a toolkit of signals that direct the complex to various cellular compartments: StanfordNeuroscience News
- Outer mitochondrial membrane (TOM70 signal)
- Processing bodies or p-bodies (DCP1a signal)
- Stress granules (G3BP1 signal)
- Telomeres (TRF1 signal)
- Nuclear stress bodies
- Neurite tips and growth cones Nature
Inducible Control Systems: To provide temporal control over RNA localization, the team incorporated inducible elements. Light-activated variants (paCas13) enable optogenetic control, while chemical induction systems allow small molecule regulation of transport. Nature +2
Breakthrough Performance Metrics
The Stanford team’s results exceeded expectations across multiple parameters:
Growth Enhancement: The most striking result was a 50% increase in neurite growth within 24 hours when growth-promoting RNAs were delivered to neurite tips. Neuroscience NewsEurekAlert! This represents one of the largest single-intervention effects reported for promoting neuronal growth. Neuroscience News +2
Spatial Precision: The system successfully delivered RNAs across the entire length of neurons, including transport over meter-long distances in cultured motor neurons. Real-time imaging confirmed precise subcellular localization. PubMed
Functional Validation: Critically, transported RNAs remained functional. The team demonstrated local protein synthesis from relocated mRNAs, confirming that the transport process preserves RNA integrity and translational competence. PubMed
Multiplexing Capability: A single dCas13 protein can process multiple guide RNAs, enabling simultaneous targeting of different RNA species to various cellular locations. Science
Molecular Discoveries Through CRISPR-TO
The technology enabled several fundamental discoveries about neuronal cell biology:
β-actin mRNA Dynamics: Relocalization of β-actin mRNA to neurite tips enhanced formation of filopodial protrusions—finger-like extensions critical for axon guidance and synapse formation. This demonstrated that RNA localization directly controls neuronal morphology. PubMedStanford
Stmn2 as a Growth Driver: Through systematic screening, the team identified Stathmin-2 (Stmn2) mRNA as a potent driver of neurite outgrowth. Stanford Stmn2 encodes a microtubule-regulating protein, and its loss is associated with ALS progression. PubMed
Ribosome Co-transport: Surprisingly, the team found that ribosomes co-transport with relocated mRNAs, suggesting that entire translation units can be mobilized within neurons. This finding has profound implications for understanding local protein synthesis. PubMed
Bidirectional Transport: The system demonstrated both anterograde (cell body to periphery) and retrograde (periphery to cell body) RNA transport, providing unprecedented control over RNA trafficking dynamics. Nature
Benefits and Transformative Potential
Advantages Over Existing Technologies
CRISPR-TO offers multiple advantages that position it as a potentially transformative therapeutic platform:
Reversibility and Safety: Unlike DNA editing, RNA targeting provides temporary modifications that can be adjusted based on patient response. This reversibility is particularly valuable for neurological applications where permanent changes carry substantial risks.
Spatial Precision: No existing technology matches CRISPR-TO’s ability to deliver therapeutic molecules to specific subcellular locations. This precision is crucial for neurons, where different compartments have distinct functional requirements. Stanford
Rapid Action: The 24-hour timeframe for observable effects contrasts sharply with gene therapy approaches that may take weeks or months to show benefits. Stanford For acute injuries or rapidly progressing diseases, this speed could be life-saving.
Preservation of Cellular Function: By working with endogenous RNAs rather than introducing foreign genes, CRISPR-TO maintains normal cellular regulation while enhancing specific functions where needed.
Platform Versatility: The modular nature of the system allows rapid adaptation to different therapeutic targets. Changing the guide RNA sequence redirects the system to new RNA targets without requiring protein engineering.
Clinical Applications and Disease Targets
The technology’s potential applications span numerous neurological conditions:
Amyotrophic Lateral Sclerosis (ALS): ALS involves progressive motor neuron degeneration partly due to failed RNA transport. CRISPR-TO could restore delivery of essential RNAs like Stmn2 to motor neuron terminals, potentially slowing or reversing disease progression. Neuroscience NewsScience Advances
Spinal Muscular Atrophy (SMA): This genetic disorder affects motor neurons due to insufficient SMN protein. Nih CRISPR-TO could enhance local translation of SMN mRNA at neuromuscular junctions where it’s most needed. Stanford +2
Spinal Cord Injury: Following traumatic injury, neurons attempt to regenerate but fail due to insufficient growth signals at injury sites. Targeted delivery of growth-promoting RNAs could enhance regeneration.
Alzheimer’s Disease: Synaptic dysfunction precedes neuronal death in Alzheimer’s. CRISPR-TO could deliver RNAs encoding synaptic proteins directly to affected synapses, potentially preserving cognitive function. Stanford
Parkinson’s Disease: Dopaminergic neurons in Parkinson’s show impaired RNA transport. Restoring normal RNA localization could protect these neurons from degeneration.
Peripheral Neuropathies: Diabetic neuropathy and chemotherapy-induced peripheral neuropathy involve axonal dysfunction that could benefit from enhanced local RNA delivery.
Broader Scientific Impact
Beyond therapeutic applications, CRISPR-TO provides an unprecedented research tool:
Fundamental Neuroscience: Researchers can now manipulate RNA localization to understand how spatial organization contributes to neuronal function, a previously intractable question.
Drug Discovery: The platform enables screening for RNAs that promote regeneration or protect against degeneration, accelerating identification of therapeutic targets.
Disease Modeling: By disrupting normal RNA localization, researchers can model diseases in culture systems more accurately than genetic knockouts alone.
Biomarker Development: Patterns of RNA mislocalization could serve as early disease indicators, enabling earlier intervention.
Real-World Implementation and Case Studies
Laboratory Validation Studies
The Stanford team’s initial validation focused on cultured neurons from mice, providing proof-of-concept data:
Primary Neuron Cultures: In dissociated cortical and motor neuron cultures, CRISPR-TO successfully redirected multiple RNA species. Live-cell imaging tracked RNA movement in real-time, confirming precise targeting. Stanford +2
Injury Models: Neurons subjected to mechanical injury showed enhanced regeneration when growth-promoting RNAs were delivered to injury sites. The 50% growth enhancement was consistent across multiple experimental replicates. StanfordNeuroscience News
Disease Models: In cellular models of ALS using patient-derived induced pluripotent stem cells (iPSCs), CRISPR-TO restored RNA transport deficits, suggesting therapeutic potential.
Comparative Efficacy Studies
When compared to existing approaches, CRISPR-TO showed superior performance:
vs. Growth Factor Treatment: While neurotrophic factors like BDNF can promote neuronal growth, they act globally and can have off-target effects. CRISPR-TO’s spatial precision avoids these limitations.
vs. Gene Therapy: AAV-mediated gene delivery takes weeks to achieve expression and cannot target specific cellular compartments. CRISPR-TO acts within hours with subcellular precision.
vs. Small Molecule Drugs: Pharmacological approaches to enhance regeneration show modest effects and poor specificity. CRISPR-TO’s targeted approach could provide greater efficacy with fewer side effects.
Translation Pathway to Clinical Application
The path from laboratory to clinic involves several critical steps:
Delivery System Development: Current work focuses on developing brain-penetrant delivery vehicles. Jennifer Doudna’s team received $1.25 million from the Rett Syndrome Research Trust to develop lipid nanoparticles (LNPs) for brain delivery of CRISPR components. InnovativegenomicsInnovativegenomics
Safety Studies: Comprehensive toxicology studies must evaluate potential off-target effects, immune responses, and long-term consequences of RNA relocalization.
Efficacy Validation: Animal models of neurological diseases will test whether laboratory results translate to therapeutic benefits in complex organisms.
Manufacturing Scale-Up: Clinical application requires GMP-grade production of CRISPR components, guide RNAs, and delivery vehicles at scale.
Regulatory Approval: The FDA has shown openness to CRISPR therapies, as evidenced by Casgevy approval, but neural applications face additional scrutiny due to the sensitive nature of brain interventions. The Stanford Daily +2
Early Clinical Development Programs
While CRISPR-TO specifically has not yet entered clinical trials, related CRISPR programs provide a roadmap:
Editas Medicine’s EDIT-301: This CRISPR therapy for sickle cell disease demonstrates the feasibility of ex vivo CRISPR applications, though in vivo neural delivery remains more challenging.
Intellia Therapeutics’ NTLA-2001: The first in vivo CRISPR therapy to enter clinical trials targets the liver, providing insights into systemic delivery challenges.
CRISPR Therapeutics’ CTX001: Now approved as Casgevy, this therapy’s success paves the regulatory pathway for future CRISPR applications. The Stanford Daily +4
Challenges, Limitations, and Ongoing Solutions
Technical Hurdles
Despite its promise, CRISPR-TO faces significant technical challenges:
Delivery Across the Blood-Brain Barrier: The brain’s protective barrier excludes most large molecules, including CRISPR proteins. Innovativegenomics Current viral vectors show limited efficiency and cell-type specificity. Solutions under development include:
- Engineered AAV variants with enhanced brain penetration
- Lipid nanoparticles designed to cross the blood-brain barrier
- Focused ultrasound to temporarily open the barrier
- Intranasal delivery to bypass the barrier entirely StanfordInnovativegenomics
Efficiency in Non-Dividing Neurons: Mature neurons present unique challenges for CRISPR delivery and function. Unlike dividing cells, neurons cannot dilute out CRISPR components through cell division, raising concerns about long-term protein expression and potential toxicity. Nih
Scalability for Human Applications: Mouse neurons used in initial studies are orders of magnitude smaller than human neurons. Scaling RNA transport over the meter-long axons found in human motor neurons presents unprecedented challenges.
Safety Considerations
Comprehensive safety evaluation remains paramount:
Off-Target Effects: While CRISPR-TO avoids DNA cutting, unintended RNA binding could disrupt normal cellular processes. Advanced computational tools and experimental validation help minimize these risks. NCBIScience
Immune Responses: Bacterial Cas proteins may trigger immune responses, particularly with repeated administration. Protein engineering to reduce immunogenicity and immunosuppressive strategies are under investigation.
Cellular Stress: Overexpression of CRISPR components can stress cells. The Stanford team addressed this by optimizing expression levels and using inducible systems for temporal control. Nature
Long-Term Effects: The consequences of chronically altered RNA localization remain unknown. Extended preclinical studies must evaluate potential adverse effects over months to years.
Regulatory and Ethical Frameworks
The regulatory landscape for neural CRISPR applications continues to evolve:
FDA Guidance: The FDA has indicated willingness to consider CRISPR therapies but requires extensive safety data for neural applications. The irreversible nature of neural interventions demands higher safety standards than systemic therapies.
International Harmonization: Different countries have varying regulations for genetic therapies. Achieving global consensus on neural CRISPR applications remains challenging but necessary for widespread adoption.
Ethical Considerations: While CRISPR-TO avoids germline modification concerns, questions remain about enhancement versus treatment. The technology’s potential to enhance cognitive function beyond baseline raises complex ethical questions. Stanford
Equity and Access: Current CRISPR therapies cost millions of dollars per patient. Ensuring equitable access to neural CRISPR treatments, particularly for rare diseases, requires innovative funding and delivery models.
Current Research Addressing Limitations
Multiple research groups work to overcome these challenges:
Delivery Innovations: UC Berkeley’s Innovative Genomics Institute leads development of novel delivery systems, including engineered viruses and synthetic nanoparticles. Innovativegenomics
Safety Enhancements: Researchers engineer Cas variants with reduced off-target activity and develop sensitive detection methods for unintended effects. NCBIScience
Efficiency Improvements: Protein engineering and guide RNA optimization continue to enhance CRISPR-TO performance in neural applications.
Manufacturing Advances: Industry partnerships focus on scalable production methods to reduce costs and improve accessibility.
Future Outlook: The Next Decade of Neural CRISPR
Near-Term Developments (2025-2027)
The immediate future holds several promising developments:
Clinical Trial Initiation: Based on current progress, the first CRISPR-TO clinical trials for ALS or SMA could begin within 2-3 years. These trials will likely focus on patients with severe disease who have exhausted other options.
Expanded Preclinical Studies: Mouse and non-human primate studies will evaluate efficacy in various disease models. These studies will inform optimal dosing, delivery routes, and treatment schedules.
Technology Refinements: Continued engineering will produce more efficient, specific, and controllable CRISPR-TO variants. Machine learning approaches may accelerate optimization.
Combination Therapies: CRISPR-TO may synergize with existing treatments. For example, combining RNA delivery with neurotrophic factors could enhance regeneration beyond either approach alone.
Medium-Term Prospects (2028-2030)
As the technology matures, applications will expand:
Broader Disease Applications: Success in initial trials could enable treatment of diverse neurological conditions, from traumatic injuries to neurodevelopmental disorders.
Personalized Medicine: Patient-specific guide RNAs could target individual disease variants, enabling precision medicine approaches for rare neurological diseases. NPR
Preventive Applications: For patients with genetic risk factors, prophylactic CRISPR-TO treatment could prevent disease onset or slow progression.
Enhanced Delivery Systems: Next-generation delivery vehicles may enable non-invasive, repeated administration for chronic disease management.
Long-Term Vision (2030 and Beyond)
The ultimate potential of neural CRISPR technology extends beyond current imagination:
Integrated Neural Interfaces: Combination with brain-computer interfaces could enable real-time monitoring and adjustment of RNA delivery based on neural activity patterns.
Regenerative Medicine: CRISPR-TO could become standard treatment for spinal cord injuries, potentially restoring function thought permanently lost.
Cognitive Enhancement: While controversial, the technology’s ability to enhance neural function could address age-related cognitive decline or enhance human capabilities.
Systems-Level Interventions: Rather than targeting single RNAs, future systems could orchestrate complex RNA networks to fundamentally alter neural circuit function.
Convergence with Other Technologies
CRISPR-TO’s impact will be amplified by convergence with other advancing fields:
Artificial Intelligence: AI-driven design of guide RNAs and prediction of optimal targeting strategies could accelerate therapeutic development.
Synthetic Biology: Engineered RNA cargoes with enhanced functions could provide capabilities beyond natural RNAs.
Nanotechnology: Precision nanodevices could provide unprecedented control over RNA delivery and release.
Stem Cell Therapy: Combining CRISPR-TO with cell replacement strategies could enhance integration and function of transplanted neurons.
The Broader Scientific Revolution
Establishing Spatial RNA Medicine
Stanford’s CRISPR-TO represents more than a single technology—it establishes an entirely new therapeutic paradigm. Spatial RNA medicine recognizes that location matters as much as presence for cellular RNAs. Addgene +2 This conceptual shift parallels other revolutionary changes in medicine:
Just as antibiotics transformed treatment of bacterial infections by targeting specific pathogens, spatial RNA medicine could transform treatment of neurological diseases by targeting specific cellular dysfunctions. The ability to deliver therapeutic RNAs precisely where needed, when needed, with reversible effects, provides unprecedented therapeutic control.
Transforming Neuroscience Research
Beyond therapeutic applications, CRISPR-TO revolutionizes basic neuroscience research. For decades, neuroscientists have known that RNA localization is critical for neural function but lacked tools to manipulate it precisely. CRISPR-TO fills this gap, enabling researchers to:
Test Causality: By altering RNA localization, researchers can determine whether mislocalization causes disease or merely correlates with it.
Map Functions: Systematic relocalization of different RNAs reveals their specific roles in various cellular compartments.
Understand Dynamics: Real-time control over RNA position enables study of dynamic processes like synaptic plasticity and axon guidance.
Model Disease: Precise recreation of disease-associated RNA mislocalization provides superior disease models for drug discovery.
Implications for Medicine and Society
The success of CRISPR-TO could catalyze broader changes in medicine and society:
Precision Medicine: The technology exemplifies truly personalized medicine—treatments tailored not just to genetic variants but to specific cellular dysfunctions in individual patients.
Reduced Healthcare Burden: Effective treatments for neurodegenerative diseases could dramatically reduce healthcare costs and improve quality of life for millions.
Ethical Evolution: Society must grapple with questions about enhancement versus treatment, equitable access, and the definition of “normal” neural function.
Scientific Collaboration: The interdisciplinary nature of CRISPR-TO—combining molecular biology, neuroscience, engineering, and medicine—models future scientific endeavors.
Expert Perspectives and Future Directions
Leading Voices in the Field
The scientific community has responded with both excitement and measured caution to CRISPR-TO’s potential:
Dr. Stanley Qi, the technology’s co-creator, emphasizes its transformative potential: “CRISPR is not merely a tool for research. It’s becoming a discipline, a driving force, and a promise that solves long-standing challenges from basic science, engineering, medicine, and the environment.” Welcome to Bio-X +2
Dr. Jennifer Doudna, CRISPR pioneer and Nobel laureate, highlights the critical importance of delivery: “Enabling brain delivery will not only be crucial for treating Rett syndrome, but for a wide range of brain disorders and diseases. How we can achieve in vivo genome editing, I increasingly think this is the bottleneck in this field.” Harvard Gazette +3
Dr. Kate O’Connor-Giles from Brown University provides a balanced perspective: “While there is also potential to use CRISPR to cure neurological disease, this needs to be balanced against the risks. The delivery of CRISPR components to cells in the brain presents a major challenge.” Brown University
Dr. Martin Kampmann from UCSF emphasizes the research potential: “With this technology, we can take skin or blood cells from a patient with a neurodegenerative disease, turn them into neurons, and figure out which genes control the cellular defects associated with the disease.” NatureScienceDaily
Critical Research Priorities
The scientific community has identified several critical priorities for advancing CRISPR-TO:
Delivery System Optimization: Developing brain-penetrant delivery vehicles remains the highest priority. Success here would unlock the technology’s full therapeutic potential.
Safety Validation: Comprehensive studies must evaluate long-term effects of RNA relocalization across different cell types and disease states.
Mechanism Refinement: Understanding exactly how CRISPR-TO interfaces with cellular transport machinery will enable rational improvements.
Clinical Translation: Establishing manufacturing processes, quality controls, and regulatory pathways for clinical application requires sustained effort.
Ethical Guidelines: Developing consensus on appropriate uses of neural enhancement technology demands proactive engagement with ethicists, policymakers, and the public.
Funding Landscape and Support
Despite recent challenges, funding for neural CRISPR research continues from multiple sources:
Federal Support: While the BRAIN Initiative faces budget cuts (from $680 million in 2023 to $321 million in 2025), targeted programs continue supporting CRISPR development. STAT +2 The NIH recently awarded $40 million to Yale for novel CRISPR delivery platforms. Yale
Private Foundations: Organizations like the Rett Syndrome Research Trust provide crucial funding for specific applications. Their $1.25 million grant to Doudna’s team exemplifies disease-focused support. InnovativegenomicsInnovativegenomics
Industry Investment: Companies like CRISPR Therapeutics, Editas Medicine, and Intellia Therapeutics continue investing in neural applications despite broader market challenges. Yahoo Finance
International Collaboration: Global partnerships, particularly with institutions in Europe and Asia, provide additional resources and expertise.
Conclusion: A New Chapter in Neural Medicine
Stanford’s CRISPR-TO technology represents far more than an incremental advance—it opens an entirely new chapter in neural medicine. By transforming CRISPR from a cutting tool into a delivery system, researchers have created possibilities that seemed impossible just years ago. StanfordNeuroscience News
The ability to achieve 50% improvement in neurite growth within 24 hours through precise RNA delivery demonstrates the technology’s transformative potential. Stanford +2 For patients with ALS watching their motor neurons slowly fail, or those with spinal cord injuries hoping to regain function, CRISPR-TO offers tangible hope grounded in rigorous science. Stanford
Yet this revolution is just beginning. The convergence of spatial RNA medicine with advances in delivery systems, artificial intelligence, and synthetic biology promises even greater breakthroughs ahead. As researchers overcome current limitations in brain delivery and safety validation, CRISPR-TO could become a cornerstone of 21st-century medicine.
The implications extend beyond individual treatments. By establishing spatial RNA medicine as a new therapeutic class, Stanford’s innovation provides a platform for addressing previously intractable neurological diseases. AddgeneStanford The technology’s reversibility, precision, and rapid action address fundamental limitations of existing approaches while opening new avenues for both treatment and research. Frontiers +2
As we stand at this threshold, the scientific community bears responsibility for advancing the technology thoughtfully. Safety must remain paramount, access must be equitable, and applications must be guided by ethical principles. With appropriate care and continued innovation, CRISPR-TO could fulfill its promise of transforming not just how we treat neurological disease, but how we understand the remarkable complexity of the human brain.
The molecular mailman has arrived, carrying hope in the form of precisely delivered RNA packages. StanfordNeuroscience News The next decade will reveal whether this delivery service can fulfill its promise of healing humanity’s most challenging neurological conditions. Based on current evidence, that future looks remarkably bright. Stanford